Mitigation options - Fluoride treatment technologies
NOTE: Article from the Geogenic Contamination Handbook
A range of technologies are available for the removal of fluoride from drinking water. These can be divided into three categories based on the underlying fluoride-removal process:
Contents
In the following section, we profile the technologies that are suitable for application at household and community scales for decentralised systems in developing countries. We focus on technologies that have already been successfully implemented in the field. Many other technologies exist that have been tested in the laboratory but have not yet proved successful in the field or were never pursued. It is important to remember the following: The ideal technology, suited for all types of conditions, does not exist!
The particular challenge for fluoride-removal technologies is the fluoride concentration in contaminated waters, which is roughly 50–150 times higher than arsenic concentration in arsenic-contaminated waters. This particularly affects the costs of the adsorption and precipitation/coagulation methods, as more filter materials, chemicals and maintenance are required. The choice of the most suitable technology will be influenced by a range of factors, such as the fluoride concentrations in the input water, the funds available for implementation, operation and maintenance requirements (O&M), the local availability of raw materials and whether the technology is accepted by the population. Cost issues are usually at the forefront when decisions concerning the selection of a technology are made. The life cycle costs that will need to be considered (e.g. capital expenditures, maintenance expenditures etc.) are described in more detail in IRC (2011) and on the WASHCost website. Readers interested in technologies that are mentioned here may consult several reviews on defluoridation methods, for example Fawell et al. (2006), Ayoob et al. (2008), Mohapatra et al. (2009), KEBS (2010), Bhatnagar et al. (2011) and Jagtap et al. (2012).
Adsorption (filter materials)
A widely used method for the removal of fluoride is to pass the contaminated water through a filter bed that retains the fluoride. The binding of fluoride to the surface of granular filter materials is an adsorptive process. In developing countries, most filter materials that have a high affinity for fluoride are aluminium- or calcium-phosphate-based. On the following pages, we describe two commonly used materials: activated alumina and bone char.
Activated alumina
Activated alumina (AA) is a commercially available granular form of aluminium oxide (Al2O3) that can be used as a filter medium to remove a range of contaminants, including fluoride, from water (Fig. 7.6). In contact with water, the surface of the AA becomes hydrated and forms Al(OH)3 with surface hydroxide groups (≡Al-OH). Negatively charged ions can replace the hydroxide (OH-) ion, as shown for fluoride:≡Al-OH + F- -> ≡Al-F + OH-
The strength of binding with the sites is reported by Amy et al. (2000) to be:OH- > H2AsO4- > F- > SO42- > HCO3- > Cl- > NO3-
This means that while hydroxide binds most strongly, the binding strength of fluoride is stronger than most ions in drinking water. This means that there will be little competition from these ions. The highest removal capacities using AA are achieved within the narrow pH range of 5.5–6, when the attraction of fluoride ions to the AA surface is at its greatest and interference with competing ions is minimised. At higher pH values, the bed capacity is significantly lower, and fluoride breakthrough occurs earlier (Rubel and Woolsley, 1979). Activated alumina is used in industrialised countries in municipal plants, but also in developing countries at community and household scales (see e.g. Venkobachar et al., 1997; Daw, 2004).
- Production: Activated alumina is a commercially available product.
- Fluoride Removal Efficiency: AA is highly efficient in reducing fluoride concentrations in treated water to levels below 0.3 mg/L. Fluoride removal of 85–95% can be achieved in well-maintained systems running at optimum conditions (Pickard and Bari, 2004). However, filter function is dependent on input-water quality and especially its pH. AA fluoride uptake capacity is at a maximum between pH 5.5 and pH 6, and it decreases considerably with increasing pH values. Waters with high alkalinity and high pH therefore need to be acidified before they are passed over the AA bed. There are many different types of activated alumina with different uptake capacities on the market.
- Regeneration and re-use: When the AA is exhausted, it needs to be regenerated. (The filter material may also be replaced, but regeneration is generally more cost-effective.) Regeneration is typically done by passing a sodium hydroxide solution (1–4%) over the AA bed, followed by rinsing with clean water. This results in caustic waste water rich in total dissolved solids, aluminium and fluoride, which needs treatment to remove these ions before disposal. The filter is then reactivated using sulphuric acid or CO2 gas, followed by flushing with water until the bed is at a pH of ~6. The fluoride removal capacity appears to be lower after each regeneration cycle. Complete replacement of the filter material is generally necessary after 3–5 regeneration cycles (e.g. Chauhan et al., 2007; Fawell et al., 2006), while application in South Africa has shown AA media to still be efficient after 6 or more regeneration cycles (Schoemann, 2008). A step-by-step documentation on activated alumina regeneration as carried out in villages in India is given in the UNICEF Report, “Regeneration Manual for Activated Alumina used in Domestic Defluoridation Units”.
- Costs: The initial costs of the filter material are generally relatively high, though efficient regeneration may bring down overall costs considerably. However, it will be necessary to establish a “regeneration centre” at a central location where spent media from household and community filters can be brought for regeneration.
- Advantages: High fluoride uptake capacity (at pH 5.5–6); Filter medium can be regenerated
- Disadvantages:Skilled operator needed for supervision of plant (community filter) and for centralised regeneration; Expensive filter material; not cost-effective if not regenerated; Pre-treatment necessary if pH of input water is too high
Bone char
The charring and crushing of animal bones produces a granular material that has been used successfully in several countries (e.g. Kenya, Ethiopia, Thailand) as a filter material to remove excess fluoride from drinking water. The removal of fluoride from water by bone char (BC) is an adsorptive process, allowing the exchange of fluoride ions with hydroxide ions (OH-) at the surface of the main mineral constituent of BC (the calcium phosphate, hydroxyapatite, Ca5(PO4)3OH), releasing OH- into solution: Ca5(PO4)3OH + F- ↔ Ca5(PO4)3F + OH-
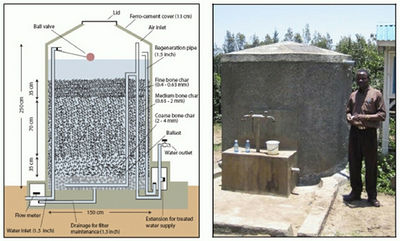
Bone char filters can be implemented at both the community and household scales (Fig. 7.7). Raw water is fed into columns or filters and is allowed to percolate through the system. Once the 1.5 mg/L fluoride threshold has been reached, the material needs to be regenerated or replaced.
- Filter Material Production: The bone material needs to be largely free of flesh before it is charred. The charring is carried out in a kiln in a low-oxygen atmosphere at a temperature of 300 to 500°C for approximately 10 days, to produce bone char with the highest fluoride removal capacity with no organic remains (CDN, 2007). If the temperature is too high, the hydroxyapatite contained in bones changes to another mineral, and the resulting bone char has a significantly reduced uptake capacity. The desired product should be grey in colour. A soot-coloured product indicates the presence of organic material, and a white-coloured product indicates that the temperature was too high. After charring, the bones are crushed to size fractions between 0.4 and 4 mm, which are washed with solution a solution of sodium hydroxide (6 g/L, pH 13) to remove remaining organic substances. The bones are then rinsed with water and acidified with CO2 gas. In Ethiopia and Kenya, large kilns capable of charring several tonnes of bone per batch are used. In Thailand, small household furnaces have been tested in which householders can produce their own bone char (Smittakorn et al., 2010). Generally, in small-scale production, it may be harder to maintain quality control of the bone char produced than in larger, standardised processes. Note: Production requires skill and may take some time to perfect. Should a faulty batch containing residual organic substances that taint the water be used, it is probable that the trust of users will be irrevocably destroyed.
- Fluoride Removal Efficiency: The fluoride uptake capacity of the filter material depends on the quality of the bone char and particle size. The smaller the particles, the higher their uptake capacity (Mjengera and Mkongo, 2002). Implementation in Kenya has shown that BC filters can reduce fluoride concentration from over 6 mg/L to less than 0.1 mg/L after filtration, with a fluoride uptake capacity of ~1.2 mg/g determined in both field and laboratory studies (Mutheki et al., 2011).
- Regeneration: Once the WHO drinking-water standard for fluoride (1.5 mg/L) or a national standard has been reached in the treated water, the filter material needs to be replaced or regenerated. Regeneration is typically done by passing a sodium hydroxide solution (0.25%–1%) through the BC bed, followed by rinsing with clean water. This results in caustic waste water rich in total dissolved solids and fluoride, which needs either to be neutralised or strongly diluted. The filter is then reactivated using CO2 gas followed by flushing with water until the effluent has a pH of ~6. The fluoride removal capacity is lower after each regeneration cycle. The caustic waste can be treated with CaCl2 or Ca(OH)2 (lime) to produce a highly insoluble solid CaF2 precipitate, which needs to be disposed of safely.
- Costs: Production of bone char is intensive in terms of infrastructure and labour. These costs and the cost of raw bones are the main contributors to the total costs. Charcoal for starting the charring process, electricity for crushing and sieving the charred bones, caustic soda for washing the bone char and bags for packing the final material are of comparatively minor importance.
- Advantages: Bones as raw material are locally available at relatively low cost; Filtered water is neutral in taste and colour (if the BC has been correctly produced); Relatively short contact time required (around 30 minutes)
- Disadvantages: Initial investments and experience needed for setting up bone char production (building of kiln etc.); The use of animal bones as a filter material is not acceptable in some regions for religious or cultural reasons; Use of low quality bone char with a high organic content might result in the treated water having an unacceptable taste; Relatively low fluoride uptake capacity (around 1.2 mg/L), which can necessitate frequent filter media replacement and lead to high transportation costs
Synthetic “bone char”: HAP Bone char essentially consists of hydroxyapatite (“HAP”, Ca5(PO4)3OH). This material can also be produced synthetically using simple raw materials (lime and phosphoric acid). Laboratory studies have shown that synthetic HAP can have a clearly higher fluoride uptake capacity than BC. Synthetic HAP is already used for fluoride removal in Germany and Italy and was used in the past in the USA. Recently, the Nakuru Defluoridation Company in Kenya has also started producing HAP and is now testing the material in the field.
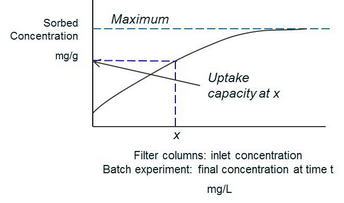
Uptake capacities of filter materials: The uptake capacity of a filter material is important, because it provides information on how long a filter material will last. The maximum uptake capacity is attained when all available sites are occupied and occurs only at high dissolved arsenic or fluoride concentrations. At lower dissolved concentrations, the amount that is sorbed is proportional to the amount in solution: Kd = Csolid/Csolution where Kd is the distribution coefficient, and Csolid and Csolution are the solid-phase and dissolved fluoride or arsenic concentrations, respectively. Thus the uptake capacity will be high at higher inflow concentrations. The uptake capacity is also influenced by solution pH, time and temperature.
!! Handling acids and bases !!: The regeneration of filter materials is usually carried out using sodium hydroxide and concentrated acids, such as sulphuric acid, for neutralisation. The handling and storage of such chemicals requires occupational health training and skills development, careful supervision and strict enforcement of rules and regulations.
Guidelines: Wear safety goggles to avoid permanent damage of the eyes when working with acids and bases. Wear suitable clothing that will protect you against spilled chemicals. Hard-soled, covered footwear must be worn at all times. Wear gloves to protect your hands. In case of spills, wash chemicals from skin straightaway.
- Wash your hands and face quickly and thoroughly whenever they come into contact with a chemical.
- If you receive a chemical burn from an acid or base, immediately wash the burned area with large quantities of water.
- Chemicals spilled over a large part of the body require immediate action. Remove all contaminated clothing and rinse with water. Do not use creams or lotions, etc. Get medical attention.
Note: If you wear contact lenses, they must be removed for effective cleansing. It is better to wear glasses in case of a spill. Work in well-ventilated surroundings to avoid inhaling of toxic fumes. Acid fumes in particular can cause permanent damage to the lungs. Always pour concentrated acids into dilute solutions or water and never the other way round. Heat is generated by the mixing process, and by controlling the amount of acid in the mixture, you can prevent the temperature from rising too much. Quick mixing can cause the mixture to boil and splash the surroundings. Further Reading: National Research Council (1995) Prudent practices in the laboratory: Handling and disposal of chemicals. Washington, DC: The National Academies Press.
Precipitation and coagulation
Fluoride can be removed from solution by precipitation and coagulation processes, followed by the settling (or flotation) of the precipitates. This usually involves the addition of chemicals that act as precipitating agents. Established techniques involving precipitation or coagulation include the Nakuru technique, the Nalgonda technique and electrocoagulation.
Contact precipitation (the Nakuru Technique)
The contact precipitation technique is also a filter method and works by adding calcium (Ca) and phosphate (PO4) compounds to untreated water, with fluoride concentrations being reduced by both sorption and precipitation reactions when the fluoride comes into contact with hydroxyapatites. Bone char provides a surface for the precipitates to form.
One method, implemented in Tanzania, is to add CaCl2 and NaH2PO4 to the water. These dissolve, releasing Ca and PO4. The resulting solution is then passed through a bone char bed (Dahi, 1996). It is relatively high in maintenance, as frequent addition of chemicals is required.
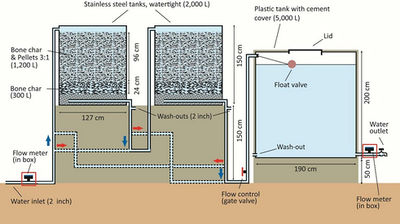
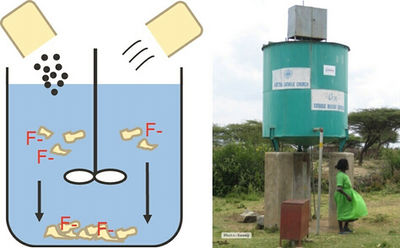
To combat this drawback, another contact precipitation approach has been developed and successfully implemented by the Nakuru Defluoridation Company (formerly the Water Quality Group of the Catholic Diocese of Nakuru (CDN WQ)) in Kenya, involving the production of calcium phosphate pellets, which slowly release Ca and PO4 when in contact with water (Fig. 7.9). This technology is known as the Nakuru Technique. The water passes through a pellet and BC mixture (3:1 ratio) and then through a bone char bed. The Nakuru Technique has successfully been implemented in fluoride removal filters in Kenya and Ethiopia (Fig. 7.10). In the following paragraphs, we will describe this method in more detail.
- Filter Material Production: To the authors’ knowledge, Ca-PO4 pellets for use in fluoride removal filters are currently only produced by NDC in Kenya. Pellets are produced in a cement mixer using Ca(OH)2, Kynofos21 (a commercially available Ca-PO4 mixture sold as animal feed) and bone dust as raw materials. Subsequent curing, washing and drying steps follow. Readers that are interested in more details should contact the Nakuru Defluoridation Company Ltd. For details on bone char production, see the “Bone Char” section in this document.
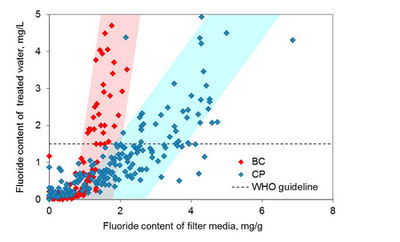
- Fluoride Removal Efficiency: Monitoring has shown that the fluoride uptake capacity of a bone char filter can be increased up to threefold, to 2–4 mg/L, when Ca-PO4 pellets are added (Korir et al., 2009; Mutheki et al., 2011) (see Fig. 7.11). The fluoride removal efficiency of the Nakuru Technique is highly dependent on the flow rate. The filters have to be designed in a way that allows the water to stay in contact with the filter medium for a long time (at least 3 hours).
- Regeneration and Disposal: Regeneration of contact precipitation filter material is not possible. It therefore needs to be replaced when the pellets are exhausted and fluoride breakthrough occurs (>1.5 mg/L). Whether spent filter material could be valuable as a phosphate fertiliser to increase crop yields is still being investigated. Preliminary research has shown that spent filter material has a lower fluoride content than commercially available fertilisers and similar phosphate availability (Hukari, 2011).
- Costs: A clear advantage over regular bone char systems is that the filter medium lasts longer, thereby reducing replacement and transportation requirements. On the other hand, the pellet costs depend highly on the cost of the calcium phosphate used for its production. If phosphate prices increase in future, the costs for pellet production will rise as well. The filter material costs (without regeneration for BC) for treating water with an initial fluoride content of 5 mg/L in Kenya are currently around 2.5 USD/m3 for CP and 4.2 USD/m3 for BC (Mutheki et al., 2011).
- Advantages: Prolonged lifespan of filter material in comparison to filters containing only bone char; Non-toxic raw materials; Research suggests that the spent medium can be reused as fertiliser
- Disadvantages: Regeneration of the filter medium is not possible; Fluoride removal efficiency is highly dependent on the flow rate, which makes its application in household filters difficult; Pellets used in the Nakuru Technique are not widely available commercially, as they are currently produced only by NDC in Kenya; Skill and experience are needed for pellet and bone char production; Kynofos21 (calcium phosphate raw material) might not be available locally and would have to be imported, or a local alternative found
Nalgonda technique
The removal of fluoride using alum as a coagulant was first proposed in the United States in the 1930s. It was later adapted by the National Environmental Engineering Research Institute (NEERI) in India in the 1970s and named the “Nalgonda Technique” (Nawlakhe et al., 1975, Fig. 7.12). It is an alum-based coagulation-flocculation method that requires alum (aluminium sulphate, Al2(SO4)3) and lime (calcium hydroxide, Ca(OH)2): Al2(SO4)3 + 3Ca(OH)2 -> 2Al(OH)3 + 3Ca2+ + 3SO42-
Alum is first dissolved and is then added to the untreated water, forming aluminium hydroxide flocs. Fluoride binds to these flocs, which are left to settle. The dose of chemicals required depends on the quality of the raw water. Although rough dose rates exist based on theoretical models and field trials (Lyengar, 2000; UNICEF, 2008; Fawell et al., 2006), these cannot be taken as standard in every case. Field trials will therefore be necessary to determine the correct dose.
- Fluoride Removal Efficiency: The Nalgonda technique may be insufficient to reduce F- values to below 1.5 mg/L when alkalinity and fluoride values in the untreated water are high. Use of the Nalgonda technique in Tanzania only reduced fluoride concentrations to 2.1–3 mg/L in water initially containing between 8 and 12 mg/L fluoride (Dahi et al., 1996). Fluoride and alkalinity levels in raw water need to be monitored frequently, as the chemical dosage needs to be adjusted according to the quality of the inlet water.
- Disposal: Fluoride- and aluminium-rich sludge is produced, which needs to be disposed of safely, out of reach of children and animals and away from drinking-water sources, preferably landfilled. Disposal in latrines is possible if these are well separated from groundwater resources.
- Costs: The chemicals needed (alum and lime) are relatively cheap and readily available in most countries, making the Nalgonda technique an inexpensive fluoride removal method if conditions are such that fluoride guidelines are met. Additional costs for a generator need to be taken into account for community units which require an electrical stirrer.
- Advantages: Chemicals readily available in most countries; Relatively inexpensive in comparison to other technologies
- Disadvantages: Insufficient fluoride removal efficiency when concentrations in raw water are high; The method is labour intensive and requires rigorous and time-consuming operation and maintenance; Some community filter units require power for the electrical stirrer; Electrical stirrers include movable parts, which are prone to mechanical failure; Perceived taste of the treated water may be affected by high sulphate concentrations (~ 600 mg/L); Large amounts of waste are produced that are often deposited onsite
Electrocoagulation
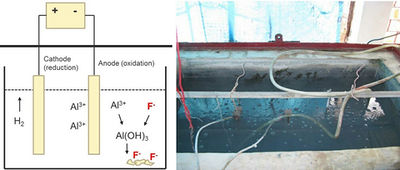
The electrocoagulation (EC) method has been used to remove fluoride and other ions from industrial wastewaters for some time (e.g. Shen et al., 2003; Hu et al., 2008) and is now increasingly receiving attention as a suitable technology for fluoride removal from drinking water in developing countries. This technology lies at the intersection of three more fundamental technologies: electrochemistry, coagulation and precipitation. The method utilises metal (e.g. aluminium) plates that act as anode and cathode. When a potential is applied to the electrodes, a current flows and Al3+ is released at the anode and reacts with water at neutral pH to form precipitate of Al(OH)3, a compound which has a high affinity for fluoride (Fig. 7.13). The resulting Al(OH)3-F flocs settle at the bottom of the solution and can be removed as sludge.
- Fluoride Removal Efficiency: Fluoride removal efficiency depends on the initial fluoride concentration, the initial pH of the influent water and the current density (Emamjomeh and Sivakumar, 2009b; Gwala et al., 2011; Ghosh et al., 2008; Zuo et al., 2008; Zhao et al., 2011). The optimum pH for fluoride removal lies between 6 and 7. Laboratory studies have shown that fluoride concentrations can be lowered from 15 mg/L to below 1.5 mg/L within 40 min (Gwala et al., 2011; Mameri et al., 1998). Field implementation in India has accomplished fluoride removal from 4.5 mg/L to below 1 mg/L within 2 hours using solar energy as an electricity source (Gwala et al., 2011).
- Disposal of Waste: The fluoride- and aluminium-rich sludge settling at the surface needs to be removed and disposed of safely, out of reach of children and animals and away from drinking-water sources. Disposal in latrines is possible if these are well separated from groundwater resources. Another possibility may be to stabilise sludge in cement or bricks.
- Costs: Electrocoagulation uses simple and readily available materials (e.g. aluminium plating). An electricity source is needed (solar panels or a generator), which can result in high initial costs and, in the case of a generator, high operational costs as well.
- Advantages: High fluoride removal efficiency (at pH 6–7); Simple system, no moving parts, No hazardous chemicals used (unless pH adjustment with acid needed); Relatively small amounts of sludge generated
- Disadvantages: High SO42- concentrations in raw water can inhibit fluoride removal; Aluminium levels in treated water may exceed the level recommended by WHO level (200 μg/L); Energy source needed (e.g. solar energy); pH may need to be controlled; Requires relatively skilled staff
Membrane methods
Membranes with fine pores can be used to separate contaminants from water physically. As the fluoride ion is very small, most membranes are not fine enough to retain it. Reverse osmosis is a technique utilising very fine membranes coupled with high pressures to remove fluoride from drinking water efficiently.
Reverse osmosis
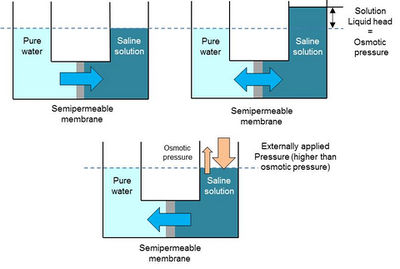
Reverse osmosis utilises a synthetic, semipermeable membrane, which allows the passage of water but not of ions or larger molecules. In principle, during the process of osmosis, water molecules move through the membrane along a concentration gradient from a high to a low dissolved salt concentration. The opposite effect is desired in the reverse osmosis process: pressure is applied on the membrane to overcome the osmotic pressure and to force water molecules from the concentrated solution to the fresh water side (Fig. 7.14). Reverse osmosis is widely applied for desalination and water purification purposes, including the removal of fluoride. More information on the principle of reverse osmosis and other membrane methods can be found in a range of documents, including Elimelech and Phillip (2011), Greenlee et al. (2009), Mulder (2000), Pontié et al. (2006) and Shannon et al. (2008).
Compared to other technologies for fluoride removal, reverse osmosis has the advantage that it removes not only ions, such as fluoride, but also pathogens (viruses, bacteria, protozoa). There are two major limitations of the reverse osmosis technology: 1 High energy requirements; 2 Membrane fouling.
Membrane fouling occurs when suspended particulate matter, colloids, bacteria and organic material are deposited on the surface of the membrane. To control fouling, a pre-filtration step or conventional pre-treatment (e.g. coagulation and disinfection) may be needed to remove the particulate, colloidal and dissolved organic matter causing the fouling. Chemical cleaning is used to restore the permeability of the fouled membranes. During reverse osmosis filtration, feed water is recirculated, and only a certain percentage (around 20–50%, depending on the system used) of the raw water ends up as treated water (permeate), the rest being waste. Reverse osmosis therefore has a high water demand and should not be used in areas of known water scarcity.
- Fluoride Removal Efficiency: Reverse osmosis can remove fluoride almost completely. Treated water can be deficient in minerals serving as essential micronutrients to humans and generally needs to undergo remineralisation before distribution.
- Costs: Reverse osmosis is a high-tech process needing skilled operators. Capital and operational costs are high. It is an energy-intensive technology, requiring the generation of high pressures. Electricity costs can therefore be substantial.
- Advantages: Efficient fluoride removal; Reduction in salinity; Additional removal of chemical contaminants and pathogens
- Disadvantages: Complex and high maintenance process; Membrane fouling needing pre-treatment and chemical cleaning; High energy consumption; High water use; Cost-intensive
References
For references, please visit the page References - Geogenic Contamination Handbook.