Difference between revisions of "Geogenic contamination - Fluoride"
m |
m |
||
| Line 1: | Line 1: | ||
| − | = Occurrence = | + | == Occurrence == |
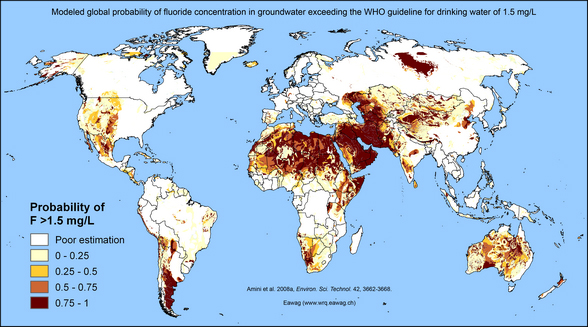
[[File:Figure 2.5.jpg|600px|thumb|right| Fig. 2.5 Modelled probability of fluoride concentrations in groundwater exceeding 1.5 mg/L (Amini et al., 2008b)]]Fluoride is the 13th most abundant element found in the Earth’s crust. Its occurrence in natural waters is closely related to the abundance and solubility of fluoride-containing minerals such as fluorite (CaF2). The fluoride concentrations of most natural waters lie below the WHO guideline value for fluoride in drinking water (1.5 mg/L, WHO, 2011). Nevertheless, elevated fluoride concentrations in groundwater are still a problem in many regions of the world (Fig. 2.5). | [[File:Figure 2.5.jpg|600px|thumb|right| Fig. 2.5 Modelled probability of fluoride concentrations in groundwater exceeding 1.5 mg/L (Amini et al., 2008b)]]Fluoride is the 13th most abundant element found in the Earth’s crust. Its occurrence in natural waters is closely related to the abundance and solubility of fluoride-containing minerals such as fluorite (CaF2). The fluoride concentrations of most natural waters lie below the WHO guideline value for fluoride in drinking water (1.5 mg/L, WHO, 2011). Nevertheless, elevated fluoride concentrations in groundwater are still a problem in many regions of the world (Fig. 2.5). | ||
| Line 12: | Line 12: | ||
| − | = Health effects = | + | == Health effects == |
Fluoride has beneficial effects on teeth at low concentrations, but excessive exposure to fluoride can have a number of adverse effects, such as dental and skeletal fluorosis, the severity of which depends on the level and duration of exposure (Gazzano et al., 2010). Worldwide, an estimated 200 million people are at risk of fluorosis. However, incomplete data make precise quantification of the global health burden of fluorosis impossible (Fewtrell, 2006). | Fluoride has beneficial effects on teeth at low concentrations, but excessive exposure to fluoride can have a number of adverse effects, such as dental and skeletal fluorosis, the severity of which depends on the level and duration of exposure (Gazzano et al., 2010). Worldwide, an estimated 200 million people are at risk of fluorosis. However, incomplete data make precise quantification of the global health burden of fluorosis impossible (Fewtrell, 2006). | ||
Revision as of 17:44, 27 April 2024
Occurrence
High geogenic fluoride concentrations in groundwater (>1.5 mg/L) are associated with a variety of geological and climatic conditions: - Granitic basements - Arid climates - Alkaline volcanic rocks
Granitic basement aquifers containing a relatively large proportion of high-fluoride minerals, such as micas, apatites or amphiboles, can yield groundwaters with elevated dissolved fluoride concentrations (e.g. in India, Sri Lanka). High fluoride concentrations can also be found in arid climates, where groundwater infiltration and flow rates are slow, and prolonged water-rock reaction times promote leaching of fluoride. Some of the highest fluoride concentrations are found in alkaline volcanic regions, such as the East African Rift Valley, where high-fluoride hyper alkaline volcanic rocks are present and fluoride is furthermore added to groundwater via high-fluoride geothermal solutions (Edmunds and Smedley, 2005). Low calcium concentrations can also lead to high fluoride concentrations in water, as fluoride is not removed from solution by the precipitation of CaF2. Such conditions are found, for example, in alkaline groundwaters whose chemistry is dominated by sodium bicarbonate. Also, groundwater associated with granitic rocks tends to contain little calcium.
Health effects
Fluoride has beneficial effects on teeth at low concentrations, but excessive exposure to fluoride can have a number of adverse effects, such as dental and skeletal fluorosis, the severity of which depends on the level and duration of exposure (Gazzano et al., 2010). Worldwide, an estimated 200 million people are at risk of fluorosis. However, incomplete data make precise quantification of the global health burden of fluorosis impossible (Fewtrell, 2006).
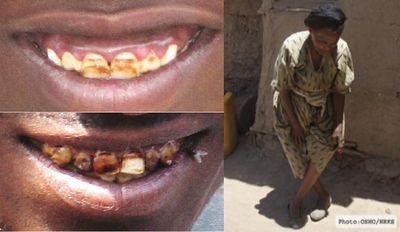
The ingestion of moderate amounts of fluoride, including that from drinking water, can lead to structural strengthening of tooth enamel and a lower rate of dental caries. The greatest decline is seen when drinking-water fluoride concentrations lie between 0.7 and 1.2 mg/L (Oszvath, 2009).This has led to the widespread fluoridation of drinking water in countries such as the USA and to the use of fluoridated toothpaste.Childhood exposure to fluoride concentrations in drinking water that exceed the WHO guideline limit of 1.5 mg/L can lead to dental fluorosis (Fig. 2.6), a condition characterised by the mottling and pitting of teeth. Dental fluorosis occurs in young children as the tooth enamel is developing (NRC, 2006). Fluoride ingestion after about the age of 8, when adult teeth have been formed (even if they have not yet erupted), will not lead to dental fluorosis. Adults with dental fluorosis must therefore have been exposed to high fluoride concentrations in their early childhood. Symptoms of mild dental fluorosis are the appearance of white striations or patches on the tooth enamel, while yellow and brown staining, pitting and chipping of the tooth enamel are symptoms of more severe fluorosis. Dental mottling is permanent, though some cosmetic treatment (bleaching, abrasion) can remove some of the visible stains.
Prolonged exposure to high fluoride concentrations over several years increases the risk of developing crippling skeletal fluorosis. This condition is characterised by pain and stiffness in the backbone and joints, accompanied by increased bone density (osteosclerosis). In its later stages, crippling deformities of the spine and joints arise, together with neurological defects, muscle wasting and paralysis (Oszvath, 2009). Studies on occupational and endemic fluorosis have shown that the extent of the symptoms is related to the duration and level of exposure and that skeletal fluorosis is at least partially reversible over a number of years. (e.g. Grandjean, 1982; Krishnamachari, 1986; Susheela and Bhatnagar, 2000). In addition to these more obvious symptoms, there are others grouped under the term “non-skeletal fluorosis”. Non-skeletal fluorosis includes a reported decrease in cognitive capacity (measured by IQ tests), lethargy, an impaired ability to concentrate and possibly even the onset of dementia (USRC, 2006). Whether these effects could be due to enzymatic changes or impaired function of the thyroid gland is unclear. Fluoride may also disturb the endocrine system, acting as an inhibitor of secretions from the parathyroid glands, which regulate extracellular calcium and phosphate concentrations. Possible effects on the gastrointestinal, renal, hepatitic and immune systems have also been reported (USRC, 2006). There has been difficulty in proving observed health effects (in scientific studies) to be the result of elevated fluoride intake, and more rigorous epidemiological studies have been recommended.
The WHO guideline value of 1.5 mg/L might not be suitable for hot, arid areas where people have a higher daily water intake (Brouwer et al., 1988). The recommended maximal daily fluoride intake for children younger than 8 years (to prevent dental and skeletal fluorosis) is 0.1 mg/day per kg of body weight (SCSEDRI, 1997, Table 3.3). For adults, a daily intake of 14 mg leads to an excess risk of adverse skeletal fluorosis, and there is evidence for increased risk of an effect on the skeleton at an intake of 6 mg/day (Fawell et al., 2006). Not only drinking water, but also cooking water and fluoride contained in food products can contribute considerably to a person’s daily fluoride uptake (Malde et al., 2011). Skeletal effects in fluorotic areas may vary in severity depending not only on the daily fluoride uptake, but also on the intake of other essential nutrients important for bone formation, such as calcium, zinc, iron and magnesium. Deficient nutrition will therefore increase the risk of bone deformation when excess fluoride is consumed (Chakma et al., 2000).